
Top Performing Drug – Invega Trinza (March Edition)
Shots:
-
In continuation to our previous series on the Top-Performing Drug of the month, based on 2021 revenue, this month we have selected Invega Trinza and prepared an engaging analysis for our readers
-
Invega Trinza (3-Months injection) is an atypical antipsychotic drug used for the treatment of schizophrenia in patients after adequately being treated with Invega Sustenna (once a month)
-
PharmaShots presents a concise take on the key features of Invega Trinza with a detailed analysis of its revenue, clinical trials, alternatives, and approvals. The report is concluded with an engaging SWOT analysis and informative KOL reviews
Active Ingredient: Paliperidone Palmitate
Dosage Forms & Strengths: Extended-release injectable suspension: 273 mg/0.88 mL, 410 mg/1.32 mL, 546 mg/1.75 mL, or 819 mg/2.63 mL
Mechanism of Action: Central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism
Originator: Janssen Pharmaceuticals
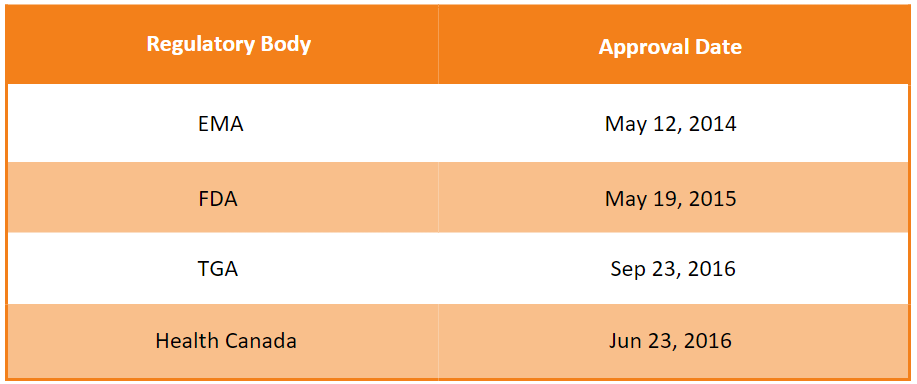
First approvals1, 2, 3, 4
The table below depicts the first approvals of Invega Trinza by different regulatory agencies.
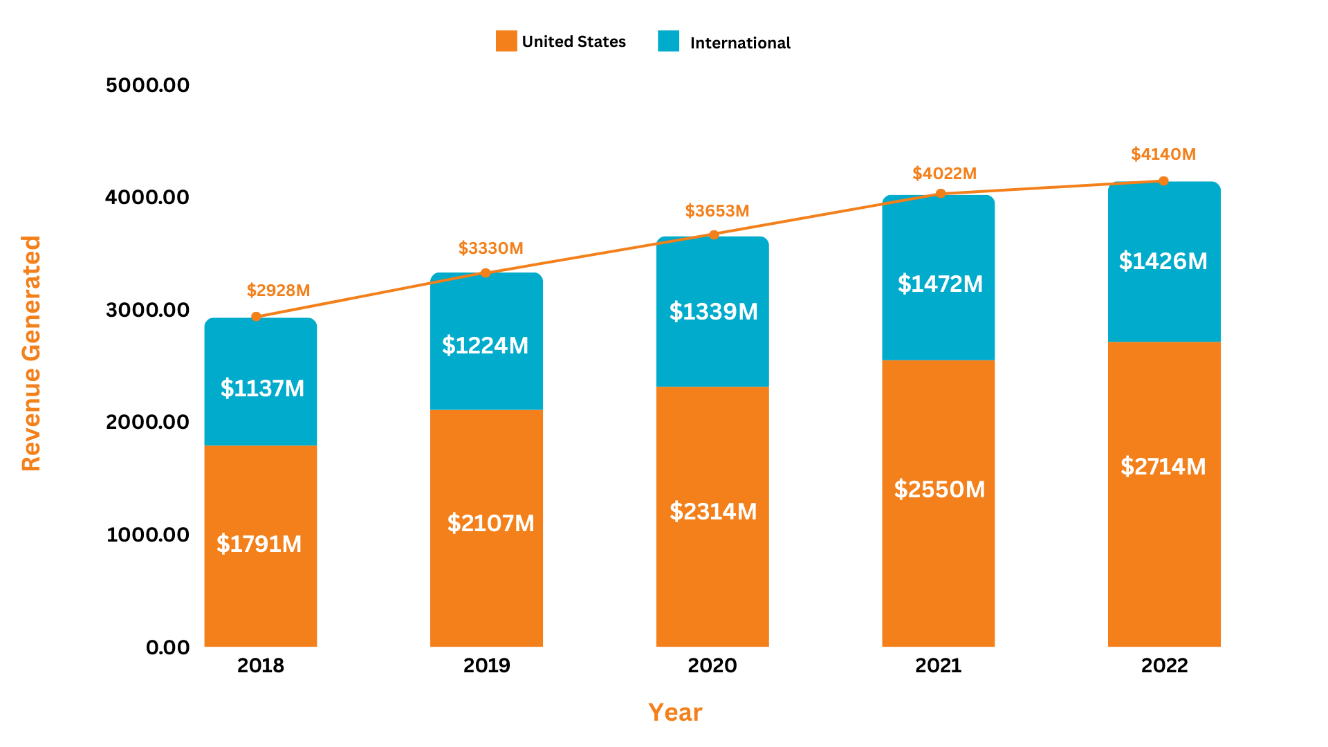
Revenue Analysis5, 6, 7, 8, 9
The annual sales of Janssen's neuroscience asset, Invega Trinza, have substantially grown over the years adding a significant amount of profit to the company’s overall revenue. In 2022, Invega Trinza/Invega Sustenna generated a total sale of $4,140M, representing a 3% increase from 2021. Over the past 5 years, the highest percentage change in the product's overall revenue was witnessed in 2019, with a 13.7% increase in sales as compared to 2018. The rise in the product's revenue was attributed to increased demand among patients suffering from schizophrenia. Moreover, in 2022, from the worldwide revenue of Invega Trinza/Invega Sustenna, i.e., $4,140M, the US market added $2,714M to the overall revenue while the international markets added $1,426M.
The following graph illustrates the revenue analysis for the last five years' sales of Invega Trinza and Invega Sustenna.
Approved Indications10
Invega Trinza is a central dopamine type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism indicated for the treatment of Schizophrenia
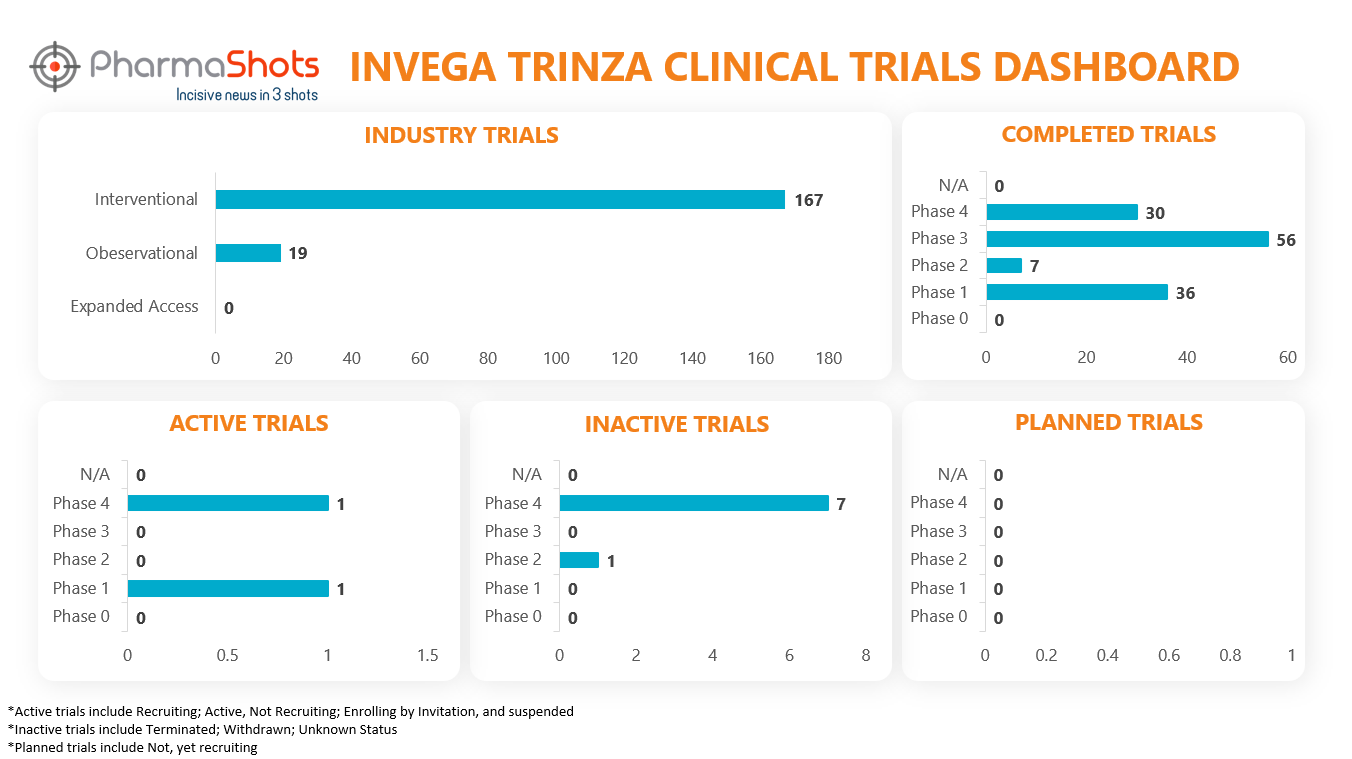
Clinical Trials Analysis11
Clinical trial analysis is vital for advancing medical science, improving patient care, and making informed decisions about the safety and efficacy of new treatments. It underpins the foundation of evidence-based medicine and regulatory approval processes, leading to better healthcare outcomes and the development of innovative therapies.
The following dashboard illustrates the clinical trials associated with Invega Trinza:
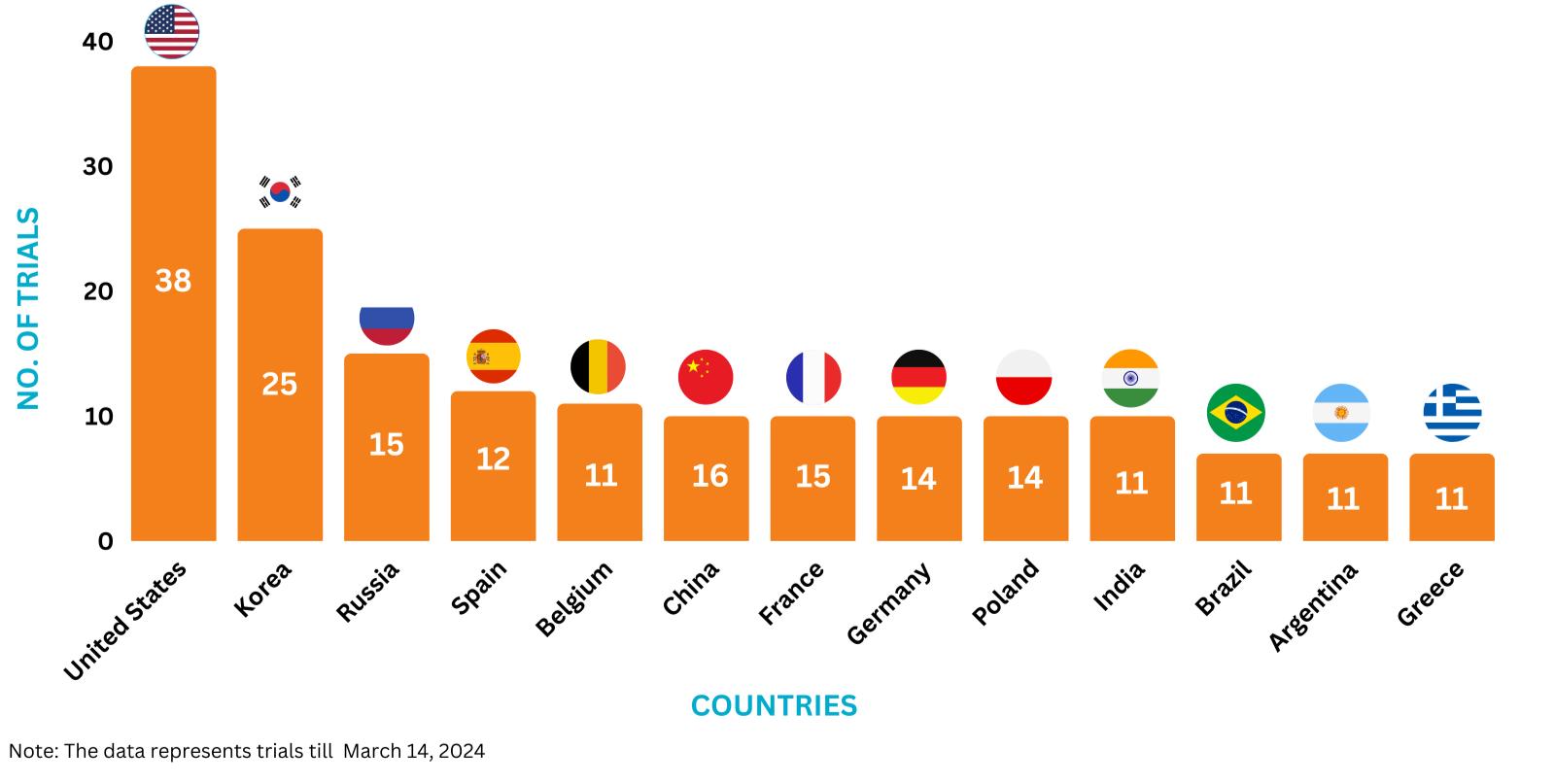
Invega Trinza Trials Representation (Country-wise)11
The graph below depicts the ongoing trials investigating Invega Trinza:
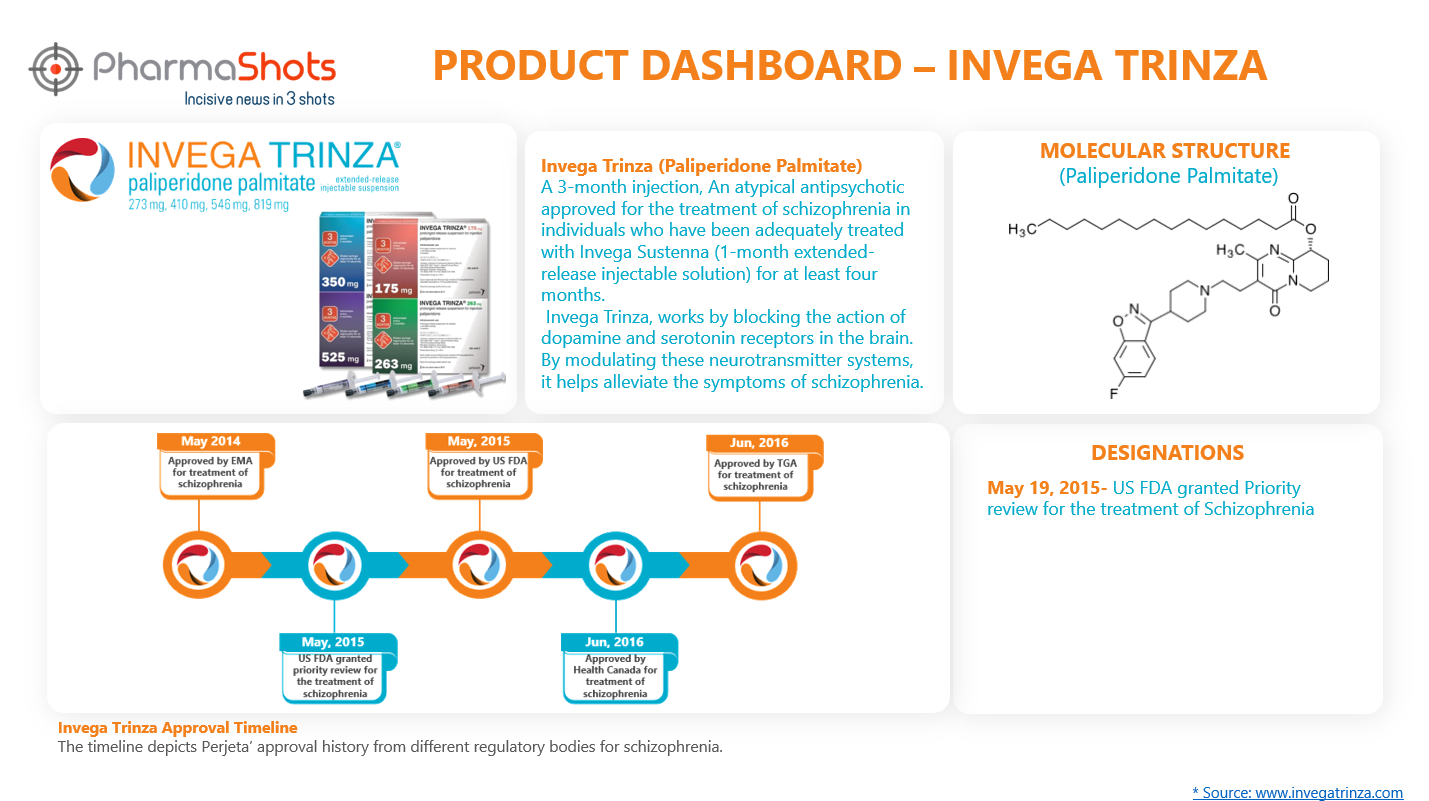
Product Dashboard10
PharmaShots presents an illustrative dashboard, highlighting essential metrics and pertinent information about Invega Trinza.
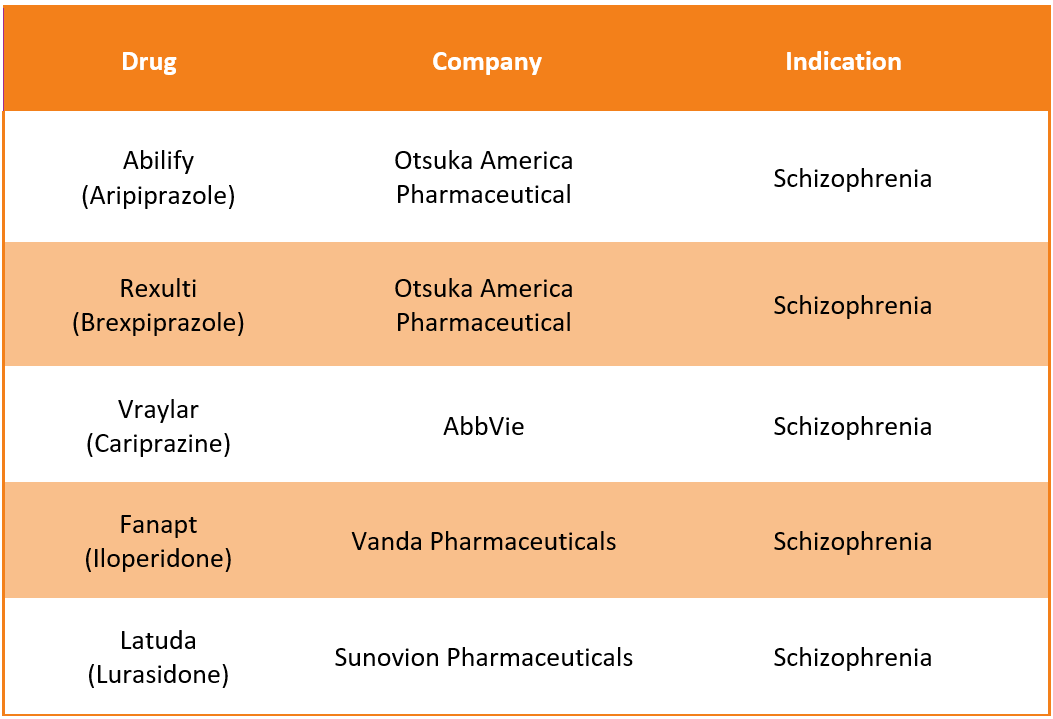
Invega Trinza Alternative Drugs12
Like Invega trinza, several alternative drugs are available in the market to treat Schizophrenia. Some of the substitute drugs for Invega Trinza include:
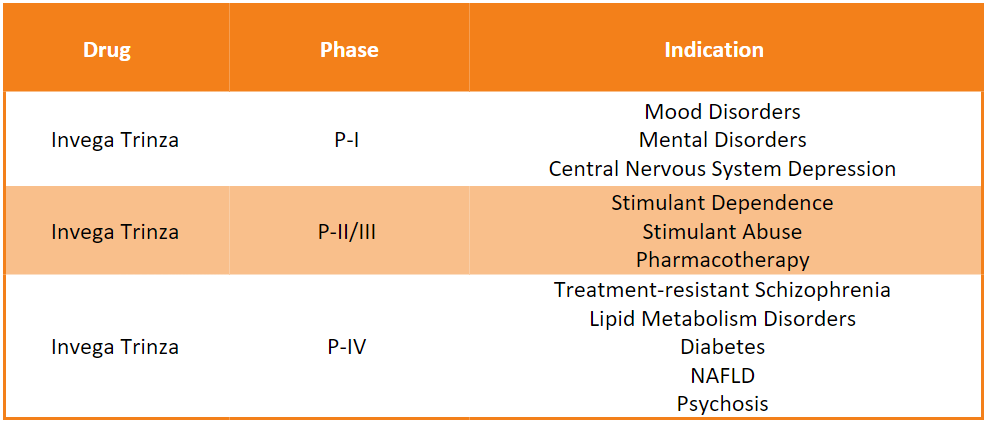
Invega Trinza Pipeline Analysis11
PharmaShots presents an extensive analysis of Invega Trinza’s pipeline, including the ongoing P-I, P-II, and P-III studies for various indications. The table below depicts an overview of these studies:
Invega Trinza SWOT Analysis
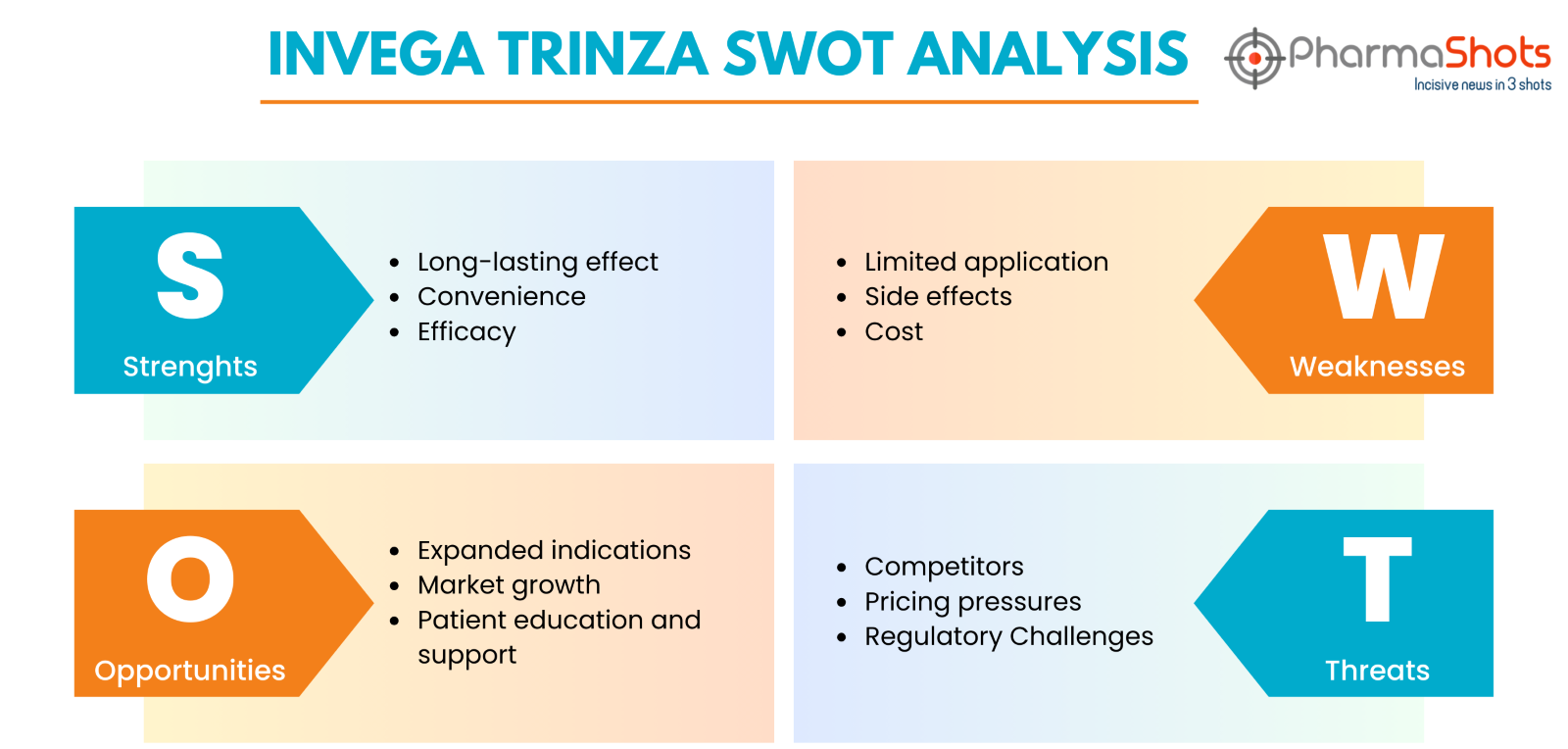
Strengths:
-
Long-lasting effect: Invega Trinza is a long-acting injectable medicine that provides sustained therapeutic benefits to schizophrenia patients for a duration of three months
-
Convenience: Its less frequent dose regimen can help patients adhere to treatment, lowering the chance of relapse
-
Efficacy: Clinical investigations have shown that Invega Trinza is useful in controlling schizophrenia symptoms, both positive and negative
Weaknesses:
-
Limited application: Invega Trinza is only indicated for the treatment of schizophrenia, which limits its market potential in comparison to drugs that treat a variety of psychiatric diseases
-
Side effects: Invega Trinza, like all drugs, can induce adverse effects such as weight gain and metabolic abnormalities, which may affect patient adherence and overall experience with treatment
-
Cost: Invega Trinza's extended-release formulation and less frequent administration may make it more expensive than other antipsychotic drugs, thus limiting availability for some patients
Opportunities:
-
Expanded indications: Janssen could investigate ways to broaden the indications for Invega Trinza beyond schizophrenia, such as other neurogenerative diseases where long-acting injectable formulations are effective
-
Market growth: With rising awareness of mental health issues and advances in psychiatric therapies, there is a growing need for novel pharmaceuticals such as Invega Trinza
-
Patient education and support: Investing in patient education and support services may help patients comprehend the benefits of long-acting injectable drugs like Invega Trinza, thereby enhancing acceptance and adherence rates
Threats:
-
Competitors: The antipsychotic drug market is highly competitive, with various choices of both oral and injectable forms. Competitors may launch similar long-acting injectable drugs, threatening Invega Trinza's market share
-
Pricing pressures: Healthcare systems and insurers may put pressure on Invega Trinza to decrease healthcare expenditures, which could result in pricing discussions or formulary limits that harm sales and profitability
-
Regulatory challenges: Changes in regulatory regulations or safety concerns may have an impact on Invega Trinza's availability or marketing, potentially resulting in setbacks or limits
Patient Stories13, 14
Patients' stories are the key resources as they provide a holistic perspective on the impact of medications on individuals' lives, improve healthcare decision-making, and contribute to the overall understanding of the drug's efficacy and safety in real-world settings. We have summarized some of the patients’ stories for Invega Trinza:
-
Schizophrenia community member 1: When diagnosed, uncertainty about the treatment was present. Fortunately, having started Invega Trinza, the right treatment option has been found, resulting in a normal life now being led
-
Schizophrenia community member 2: About a year into the journey, a treatment plan that worked was found through collaboration with doctor. Now, Invega Trinza received once every 3 months, and schizophrenia journey is proceeding well
KOL* Reviews15, 16, 17, 18, 19
-
Joseph Kwentus, MD, Precise Research Centers; Trial Investigator says, “With a dosing interval that can be measured in seasons, not days, people living with schizophrenia and their treatment teams can focus on recovery goals beyond short-term symptom control, Recovery looks different for everyone, and the long-term symptom control offered by INVEGA TRINZA™ can help patients work toward their own personal goals.”
-
Nancy Nesser, J.D., Pharm.D., OHCA Pharmacy Director says, “INVEGA SUSTENNA® has been shown to delay time to relapse and hospitalization compared to a group of oral antipsychotics, providing a significant opportunity to curb medical costs. And this, along with improved health care, is our goal for SoonerCare members.”
-
Husseini Manji, MD, Global Head, Neuroscience Therapeutic Area, Janssen R&D says, “Building on Janssen’s more than 50 years of leadership in developing innovative mental health therapies and helpful programs, this medication offers a new paradigm for treating people living with schizophrenia, after at least four months on INVEGA SUSTENNA®, patients and their doctors can seamlessly transition to INVEGA TRINZA™ for sustained symptom control with a single dose every three months.”
-
Paul Gionfriddo, president and CEO, Mental Health America says, “It’s encouraging to see continued progress in the treatment of schizophrenia, since access to a range of treatment options is a critical success factor in the treatment journey of individuals living with this disease, as both an advocate and a parent of an adult son with schizophrenia, I can attest to the importance of novel therapies that enable our loved ones to spend more time focusing on their recovery and less time worrying about taking medications.”
-
Michelle Kramer, MD, MPH, Vice President, Neuroscience Medical Affairs, Janssen says, “The inclusion of this novel evidence in the Invega Sustenna label will help adults living with schizophrenia and their healthcare providers see what’s possible with the right treatment plan and support.”
*Key Opinion Leaders (KOLs) are crucial when it comes to the launch and assessment of pharmaceutical products. At Octavus, we recognize the importance of KOLs in the industry, which is why our proficient team dedicatedly tracks their activities and provides valuable insights to the pharma fraternity.
We understand that KOL tracking and selection can be overwhelming and time-consuming. That's why we offer extensive KOL tracking services to help our clients stay ahead of the curve. Our team of experts can provide you with the latest information on KOL activities, including their opinions, publications, and affiliations.
Interested in learning more about our KOL tracking services? Don't hesitate to reach out to us at bd@octavusconsulting.com or connect@pharmashots.com. We would be more than happy to provide you with more information and discuss how our services may benefit your business.
Octavus is a dedicated consulting company that offers a one-stop market solution to life science enterprises, biopharma, MedTech, diagnostic centers, digital health companies, animal healthcare, and start-ups.
References:
Related Post: Top Performing Drug – Entyvio (February Edition)
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














